Accreditation
What is Accreditation?
Accreditation is the formal recognition of the competence of conformity assessment bodies to carry out specific conformity assessment tasks. Accreditation delivers confidence in certificates and conformity statements. It underpins the quality of results by ensuring their traceability, comparability, validity, and commutability.
What are Conformity Assessment Bodies?
Conformity Assessment Bodies include:
- Testing Laboratories (EN ISO/IEC 17025)
- Calibration Laboratories (EN ISO/IEC 17025)
- Medical Laboratories (EN ISO/IEC 15189)
- Inspection Bodies (EN ISO/IEC 17020)
- Certification Bodies (EN ISO/IEC 17065, EN ISO/IEC 17024, EN ISO/IEC17021-1)
- Validation/Verification Bodies (ISO/IEC 17029)
- Proficiency Testing Providers (ISO/IEC 17034)
- Reference Material Producers (ISO/IEC 17043)
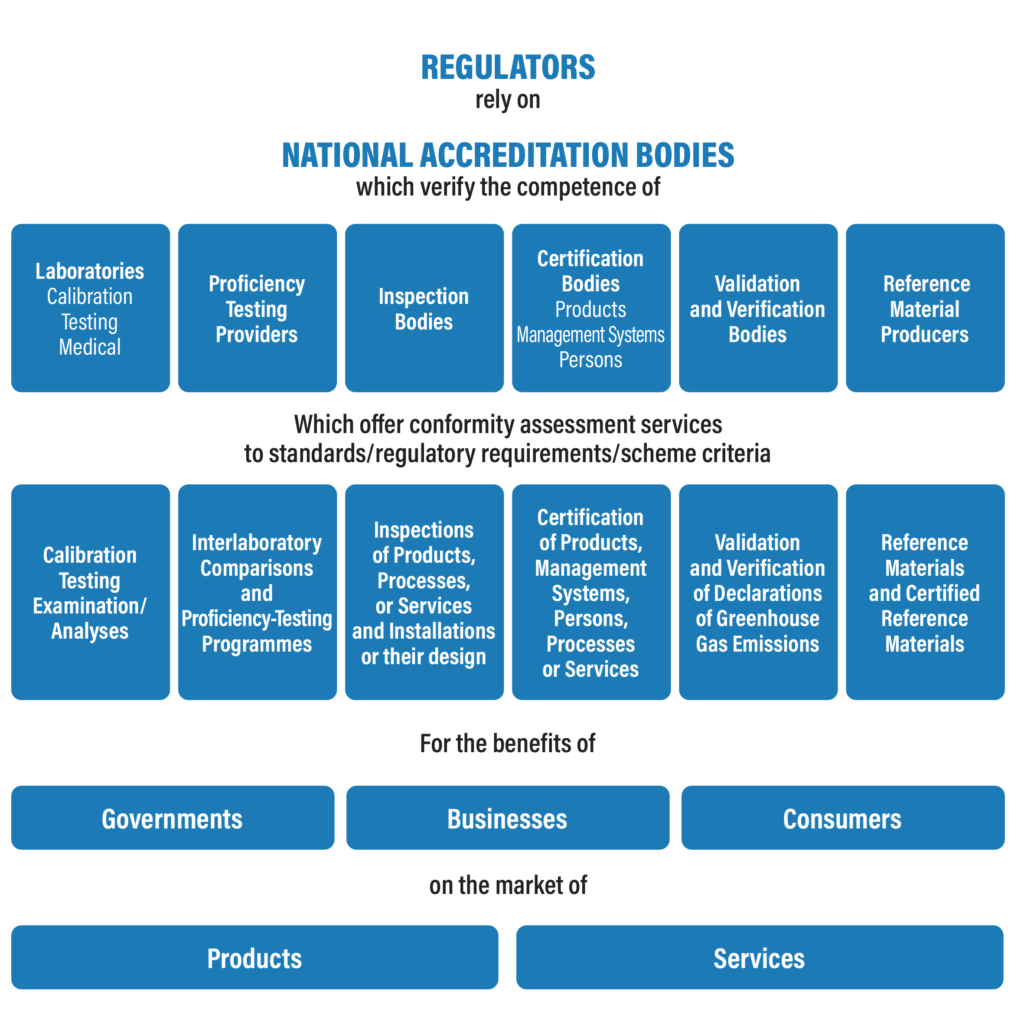
International trade relies on certificates and reports issued by competent bodies. Confidence in certificates and reports is achieved by accreditation. Accreditation is based upon a series of confidence building steps between National Accreditation Bodies (NAB’s) and Conformity Assessment Bodies (CAB’s), and the subsequent assurance given by NABs that CABs continuously maintain and enhance their competence. The latter process is on-going, as defined in accreditation standards. This assurance is achieved through on-site assessments and regular surveillance activities.
